Category: Our scienceUse this category for scientific articles that need to be added to the “Our science” overview
Use this category for scientific articles that need to be added to the “Our science” overview

Evaluating a digital tool for supporting breast cancer patients: a randomized controlled trial protocol (ADAPT)
Lidington et al., 2020; BMC. OWise is being assessed by the randomised clinical trial ADAPT at the Royal Marsden NHS Foundation Trust. Find out more here:
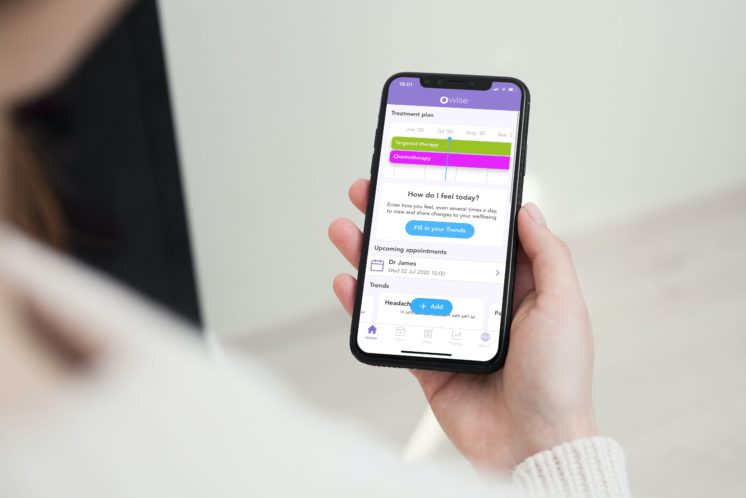
Patients’ and Health Care Providers’ Opinions on a Supportive Health App During Breast Cancer Treatment: A Qualitative Evaluation
Young-Afat et al., 2016; JMIR Cancer. Independent study analysed OWise app. Read more to know what the study found!

Evidence of the use of Mobile Apps During Treatment of Breast Cancer: systematic review
Cruz et al., 2019; JMIR. Systematic review concluded that mobile apps for women with breast cancer might be an acceptable information source that can improve patient well-being.

CE-marked: Class I Medical Device
OWise was CE-marked to comply with the European regulatory standards for safety and usage on the 7th of May, 2014.

Publicly available apps for cancer survivors: a scoping review
Adam et al., 2019; BMJ Open. Review of 151 cancer apps for individuals living with and beyond cancer.

NHS Digital approved
OWise is one in five cancer apps listed in the NHS app library.

NICE exemplar
A case study example in the NICE evidence standard framework for digital health technologies.
