
Illustration by: Marc Christoforidis
When confronted with breast cancer, you may have heard terms like genomic test being used frequently. Tumour genomic tests analyse the activity of certain genes (a gene is the basic physical and functional unit of heredity) on a sample of tumour. Although these types of tests are conducted for all kinds of diseases, in cancer they have changed how the disease is treated. That’s why, in this blog, we decided to explain the objective and the different types of genomic tests available.
What is the difference between tumour genomic and genetic tests?
Tumour genomic tests measure the expression of certain genes in a piece of tissue from a tumor. The expression of these genes can often be seen as a measure of the activity of the gene. The activity level of these genes affects the behavior of the cancer, including how likely it is to grow and spread.
Tumour genomic tests are usually ordered after surgery for people with early stage hormone receptor positive (HR-positive) and HER2 negative (HER2-) breast cancer. Generally, the main objective of these tests is to study the tumour characteristics to decide if certain treatments are needed or the risk of the cancer returning. Examples of these tests are Oncotype® and Endopredict®, which will be explained in more detail in the coming sections.1
So, what are genetic tests?
Every person is born with their own genome which contains a complete set of genetic instructions. We inherit these genetic instructions (genes) from our parents and they have all the necessary instructions to help build our bodies and allow them to grow and develop.
The sequence of the whole human genome was completed in April 2003 as part of the Human Genome Project (HGP). This information gave us the opportunity to read nature’s complete blueprint for building a human being. To learn more about the HGP, click here.2
Genetic tests are the type of tests people do when they might have an increased risk of developing breast cancer at an early age. These tests use blood, saliva or other tissue samples, to look at the genes that we inherited from our parents. With this information, we can detect variants of genes like BRCA1 and BRCA2 that are linked to the development of breast cancer.3 To learn more about the inherited risk of breast cancer, please check out our previous blog.
Another type of genetic test is called “next generation sequencing (NGS)” or “sequencing panels”. These tests look at a longer list of risk-related genes3 4 and can provide information about the vulnerabilities of the tumour. This information can be useful for doctors to choose drugs that either target or exploit these vulnerabilities. Sequencing panels are currently being developed, especially for secondary breast cancer patients.
Did you know that OWise generates a personalised treatment report as well as suggested questions for each user based on their breast cancer type? When you enter your cancer profile details (tumour size, hormone receptor status, HER2 status, BRCA1/2…) the smart software of OWise creates a report with the treatments that you are most likely to receive. These reports follow NCCN guidelines and can be very helpful for you to understand and explore your treatment plan further.
The use of all these tests in medicine is sometimes referred to as “cancer precision medicine”. Cancer precision medicine uses clinical, pathological, molecular and genomic assessments to choose the appropriate therapy for each patient at the appropriate time.5 In breast cancer, this means:
- detecting the hormone receptors (oestrogen and progesterone) and the HER2 protein;
- running genomic profiling tests like Oncotype® and Endopredict®, and
- next-generation sequencing, especially for secondary breast cancer patients.
Do you qualify for a tumour genomic test?
There are multiple genomic tests in the market. The most common ones to predict the chances of cancer coming back and the necessity of receiving chemotherapy in addition to hormonal therapy are Oncotype DX® , Endopredict®, Mammaprint® and Prosigna®.
Most of the tests are recommended after surgery to people with:
- Early breast cancer (Stage 1 and 2)
- Oestrogen receptor positive (ER+)
- HER-negative (HER-)
- No cancer cells in their lymph nodes (node-negative) or only in one to three nodes.
Please note that some genomic tests such as Mammaprint® can be used irrespective of ER and HER2 status.6
If you are unsure if you are eligible for these tests, please refer to your pathology report and/or ask your doctors (to learn more about all of the information in a pathology report, check out this blog).
How do they work?
Tumour genomic tests help individualise treatment paths further and avoid unnecessary chemotherapy treatment. They measure the expression (activity) of a list of genes. Most common genomic tests (Endopredict® and OncotypeDx®) analyse the activity of certain genes using a very common technique called “qPCR”. The analysed genes can be proliferation-associated genes, hormone receptor-associated genes, cancer-related genes… etc. With this information and other clinical characteristics such as the tumour size or the number of lymph nodes that contain cancer cells, a predictive score is calculated, which reflects the risk of cancer recurrence.
Generally, the lower the score, the lower the risk of recurrence, and vice versa. If your cancer sample has a low risk score, your cancer is not likely to return and therapy with harsh treatments (such as chemotherapy) may not be needed, whereas if your cancer sample has a high risk of recurrence chemotherapy is likely to be recommended.
It is important to note that the risk of your cancer coming back depends on many factors. There are risk assessment tools such as PREDICT 7. The PREDICT tool is freely available and it considers factors such as age, mode of detection, tumour size, grade, HER2 status, ER status, number of lymph nodes, Ki-67 status and general chemotherapy regimen to predict 5 years and 10 years survival. You might find useful going through the PREDICT tool with your doctor or nurse to discuss the different treatment options.
Most common genomic tests
 This section will provide a brief outline of the most common genomic tests available. They are all used to measure the risk of cancer recurrence within the near future, which is a factor used when considering the need for chemotherapy treatment in addition to hormone therapy. The tests analyse different genes and some assume the patient is on hormone therapy treatment in their calculations. Certain tests are designed specifically for post menopausal women. All of the tests have their individual scales and score ranges which are explored in further detail below.
This section will provide a brief outline of the most common genomic tests available. They are all used to measure the risk of cancer recurrence within the near future, which is a factor used when considering the need for chemotherapy treatment in addition to hormone therapy. The tests analyse different genes and some assume the patient is on hormone therapy treatment in their calculations. Certain tests are designed specifically for post menopausal women. All of the tests have their individual scales and score ranges which are explored in further detail below.
1. Endopredict®
Endopredict® is a genomic test that analyses the activity of 12 genes in your breast cancer cells to predict the likelihood that cancer will come back within the next 10 years.6 This test assumes you have received hormone therapy. It is given to both pre and post menopausal women, who have ER+ HER2 negative early stage breast cancer.1
It takes the activity of these genes along with other information such as the tumour size and the nodal status and introduces them into a formula to calculate a recurrence score called EPclinRisk score. This score is a number between 1.1 and 6.2 that indicates the percentage risk of recurrence during the next 10 years. The score is considered low risk if it is lower than 3.3287 as it indicates that the cancer recurrence is lower than 10%, and high risk if it is higher than 3.3287 which means that the recurrence is higher than 10%.
Your specialist will use this score, along with other information about your breast cancer to help decide if chemotherapy would be of benefit to you.
2. Oncotype DX®
OncotypeDX® is a genomic test that analyses the activity of 21 genes that can affect cancer growth, and how likely it is to spread.9 It is used to calculate the risk of recurrence within the next 10 years. OncotypeDX® assumes you have had hormone therapy treatment and is therefore helpful in determining the need for chemotherapy. This test is given to both pre and post menopausal women with stage 1 or 2 ER +, HER2- [HER2 negative] breast cancer.1
The result of the test is usually a recurrence score on a scale 0-100. For women older than 50, a score of 0-25 means that the cancer has a low risk of recurrence, while a score of 26-100 indicates that the cancer has a high risk of recurrence.10 Meanwhile, for women aged 50 and younger, a score of 0-15 means the cancer has low risk of recurrence, 16-20 low to medium risk, 21-25 medium risk and 26-100 high risk of recurrence.10 This information is used together with the tumour size and grade to evaluate if chemotherapy will be needed to reduce the chance of recurrence.
3. MammaPrint®
MammaPrint® analyses 70 genes associated with cancer spreading.11 It is mainly used to predict the risk of cancer recurrence in the next 5 to 10 years, if no hormone therapy or chemotherapy is given.1 This test can be used irrespective of ER and HER2 status, for both pre and post menopausal women with stage 1 or 2 breast cancer.11
MammaPrint® test results are calculated on a scale of -1 to +1. A score between -1 and 0 indicates a high risk of recurrence within the next 10 years, and a result between 0 and +1 indicates a low risk (less than 10%) of recurrence within the next 10 years.1 This information can be used by clinicians to determine whether chemotherapy would be a necessary treatment.
4. Prosigna® or PAM50 test
Prosigna® or PAM50 test analyses 50 genes and is used to detect breast cancer tumour subtype, and to predict risk of recurrence within the next 10 years. PAM50 stands for Prediction Analysis of Microarray 50.12 It is specially indicated for postmenopausal women with early stage ER+, HER2- breast cancer and assumes hormone therapy treatment.9 This test calculates a score based on information analysed from the 50 genes, the proliferation score, in combination with breast cancer subtype, nodal status, and tumour size. (For more information on these factors, have a read over our previous blog on pathology reports).
The score is between 0 and 100. A higher PAM50 score means that the cancer has a higher risk of recurrence and the more likely it is that chemotherapy will be a useful treatment. If cancer is not found in your lymph nodes, a score of 0-40 is considered low risk of recurrence, 41-60 is considered intermediate risk and 61-100 high risk.1 If you are lymph node positive, a score of 0-15 is considered low risk of recurrence, 16-40 intermediate and 41-100 is considered high risk.1
5. Breast Cancer Index
The Breast Cancer Index test differs from the previously mentioned tests, as it is used to evaluate the benefits of extending hormone therapy, rather than the effects of chemotherapy. It analyses the activity of 7 genes to help determine whether extending hormone therapy (from 5 to 10 years) would be beneficial.1 This test is used for women with node negative (no cancer found in lymph nodes), HR+ breast cancer. It also helps predict the risk of recurrence 5-10 years after diagnosis.10
This test provides two results: the BCI Prognostic and the BCI Predictive. BCI Prognostic estimates the risk of recurrence within the next 5 to 10 years.13 It is given in a percentage, for example 1.5% means you have a 1.5% chance of the cancer recurring. BCI Predictive calculates whether an additional 5 years of hormone therapy would be beneficial for you. It is given as a “yes” or “no”.13
Genetic tests for secondary cancer patients
Next generation sequencing (NGS) or sequencing panels are genetic tests currently being developed mainly for secondary cancer. Certain changes in genes can make you eligible for a specific treatment. An example of this is in the gene PIK3CA, which makes you eligible for the drug alpelisib. Other findings are not as direct as these, and it is the accruing of various factors that will help decide the best treatment option for you.
There are multiple companies that provide the service which can be refundable through healthcare insurance such as Guardant360, Claris, Tempus or Foundation medicine.
We know there’s a lot of information here; the OWise app can help you to stay organised and prepare for your upcoming hospital visits. Also, whichever treatment plan you and your care team decide is best for you, it is important to record and track any side effects you may experience.
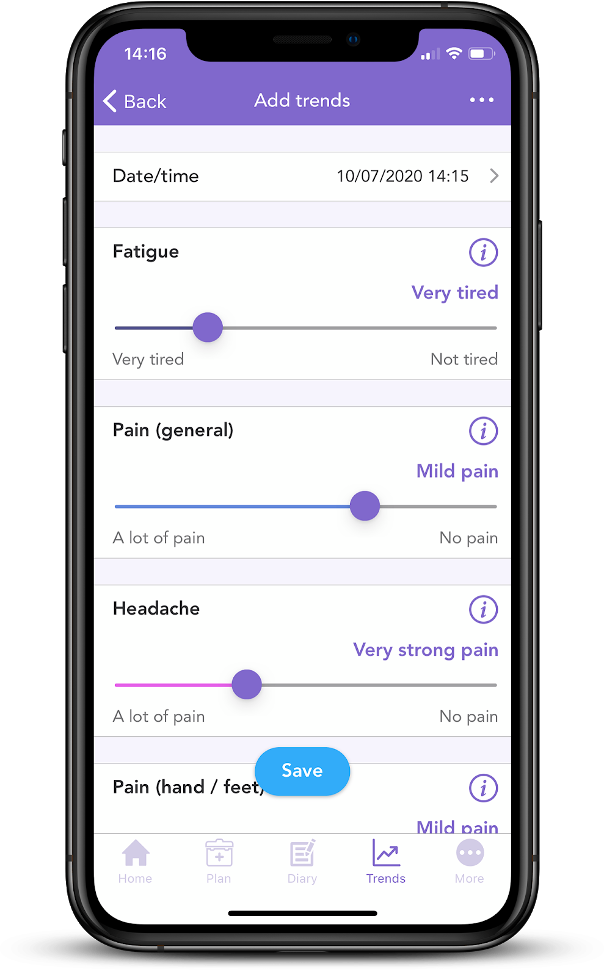
Did you know OWise app has a series of sliders which can be used to record any side effects on a daily basis? This information can be turned into weekly or monthly graphs that chart the progression of your side effects. Having this information can make communication with care teams and assessment of management strategies more accurate and detailed.
And, that’s all for tumour genomic tests! We hope that you now better understand the difference between genomic and genetic tests and can feel confident in discussions with your care team. Our aim is to make sure you are kept informed so make sure to follow our Instagram and Twitter accounts for any updates.
References
- “Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer”. nice.org.uk. 2018. https://www.nice.org.uk/guidance/dg34/chapter/3-The-diagnostic-tests
- “The human genome project”. Genome.gov, 2021 https://www.genome.gov/human-genome-project
- Lynch, J., Venne, V., Berse, B. “Genetic Tests to Identify Risk for Breast Cancer”. Seminars in Oncology Nursing. 31(2), (2015): 100-107.
- Hempel, D., Ebner, F., Garg, A, Trepotec, Z., Both, A., Stein, W., Gaumann, A., Guttler, L., Janny, W., DeGregorio, A., Hemper, L., Milani, V. “Real world data analysis of next generation sequencing and protein expression in metastatic breast cancer patients”. Scientific Reports, 10 (2020).
- Colomer R., Modenjar, R., Romero-Laorden, R., Alfranca, A., Sánchez-Madrid, F., Quintela-Fandino, M. “When should we order a next generation sequence test in a patient with cancer?” Lancet. 25 (2020).
- “Tumor Genomic Assay Tests”. Breastcancerorg. September 2020, https://www.breastcancer.org/symptoms/diagnosis/genomic_assays
- “Predict Breast Cancer”. nhs.uk. https://breast.predict.nhs.uk/tool
- Rakha, EA., Soria, D., Green, AR., Lemetre, C., Powe, DG., Nolan, CC., Garibaldi, J., Ball, G., Ellis, I. “Nottingham Prognostic Index Plus (NPI+): a modern clinical decision making tool in breast cancer”. Br J Cancer. 110(7) (2014):1688–1697.
- Ehsani, Sima, and Kari Braun Wisinski. “Genomic Testing in the Management of Early-Stage Breast Cancer.” Journal of clinical outcomes management : JCOM vol. 24,5 (2017): 229-238.
- DePolo, Jamie. “Oncotype DX Genomic Tests.” Breastcancer.org, January 2021, https://www.breastcancer.org/symptoms/testing/types/oncotype_dx
- “MammaPrint Tests.” Breastcancer.org, January 2021, https://www.breastcancer.org/symptoms/testing/types/mammaprint.
- “PAM50 (Prosigna).” Komen.org, December 2020, https://www.komen.org/breast-cancer/diagnosis/factors-that-affect-prognosis/pam50-prosigna/
- “Breast Cancer Index Test.” Breastcancer.org, January 2021, https://www.breastcancer.org/symptoms/testing/types/breast-cancer-index-test
- “Breast Cancer: Back to Basics.” Cam.ac.uk, 2012, https://www.cam.ac.uk/research/news/breast-cancer-back-to-basics
