
If you have estrogen receptor positive (ER+) breast cancer, it is likely that you are on tamoxifen. But, did you know that the effectiveness of this drug can be boosted or compromised based on everyday foods and drinks?
How does tamoxifen work?
Tamoxifen is one of the widely prescribed breast cancer treatments for premenopausal women. It works by blocking estrogen receptors in breast cancer cells, preventing estrogen from activating the cancer cells to grow further.
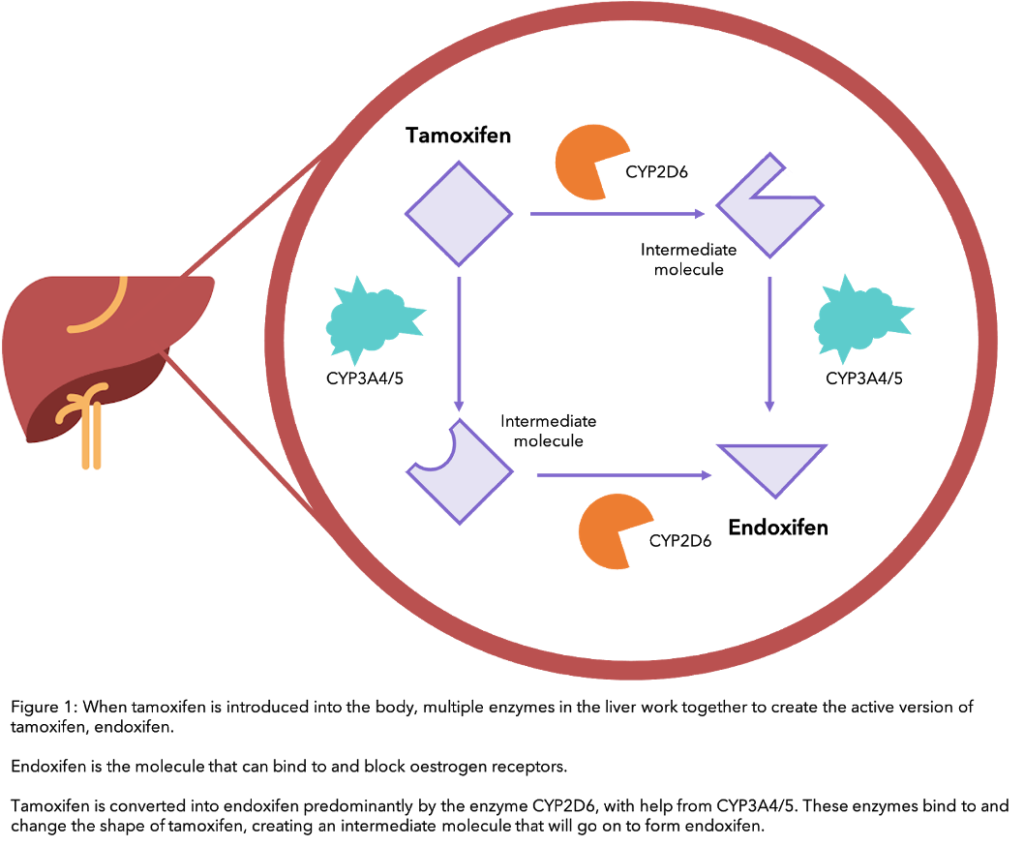
For tamoxifen to work, your body needs to process it to turn it into its active form, endoxifen. At each step in the process, a specific liver enzyme – a protein that speeds up a process within cells – is used to convert one form of the drug into another, until endoxifen is formed (see diagram). The main enzyme involved in the conversion of tamoxifen to endoxifen is called CYP2D6.
Did you know that you can track your side effects, including those most commonly caused by tamoxifen, such as hot flushes, nausea and weight gain, with OWise? You can share this data safely with your care team and loved ones and make sure you receive the best care possible. Try out the side-effects tracking and let us know if it helps you. Download the free app today.
CYP2D6 Enzyme and Diet
In order to make sure that tamoxifen is converted into its active form (endoxifen), all the liver’s CYP2D6 enzyme should be utilized. However, this enzyme is also needed to break down other substances. Therefore, these substances can “occupy” this CYP2D6, leaving less available to convert tamoxifen into endoxifen. This would essentially result in the tamoxifen you took as being labeled ineffective as the body would not be able to process it1.
Certain foods, drinks and medications can interfere with the conversion of tamoxifen to endoxifen by the CYP2D6 enzyme to different extents. If this process is altered, the effectiveness of tamoxifen in your body can also change. Although this enzyme is very important in this process, other enzymes such as CYP3A4/5 are also involved (see the diagram).
Is there anything that I should avoid eating or drinking while on Tamoxifen?
Grapefruit
Grapefruit and grapefruit juice are well-known disruptors of numerous medications 2. This is mainly due to chemicals called “furanocoumarins” present in grapefruit. They bind with the enzyme CYP3A4 3, which means that drugs such as tamoxifen cannot use the engaged CYP3A4 enzymes to convert into their active forms in the body, reducing the drug’s effectiveness. In some cases, just one grapefruit or one glass of grapefruit juice is enough to limit the effectiveness of drugs such as tamoxifen 2.
Seville oranges (often used in marmalade) also produce furanocoumarins 3, 4, so you may want to limit your intake of these as well.
Turmeric/ Curcumin
 Curcumin, the bright orange-yellow substance in turmeric, is a popular spice to ingest, especially in those undergoing cancer treatments, due to its supposed anti-cancer effects 5,6. Due to its poor absorption and rapid metabolism in the body, curcumin is often ingested in combination with black pepper, as it contains piperine, which increases the effectiveness of curcumin in the body by about 20-fold 7.
Curcumin, the bright orange-yellow substance in turmeric, is a popular spice to ingest, especially in those undergoing cancer treatments, due to its supposed anti-cancer effects 5,6. Due to its poor absorption and rapid metabolism in the body, curcumin is often ingested in combination with black pepper, as it contains piperine, which increases the effectiveness of curcumin in the body by about 20-fold 7.
Previous studies have found mixed results when it comes to whether curcumin would increase or decrease the effectiveness of tamoxifen 8,9,10. However, a recent breakthrough found an overall negative relationship between tamoxifen and curcumin 11. The study found that with curcumin consumption, the amount of active endoxifen circulating the body decreased. This amount further decreased with the additional consumption of piperine. Therefore, if you are on tamoxifen it may be best to avoid turmeric and curcumin (particularly in concentrated supplement form), as it can lower the concentration of endoxifen in the body such that it interferes with the therapeutic impact of the drug.
Black cohosh
You may have heard of black cohosh as being a herbal alternative to counteract menopausal symptoms such as hot flushes. Although some people may find it helpful, there is a lack of evidence supporting the efficacy of black cohosh for reduction of hot flushes 12.
Research in the lab has shown that black cohosh can inhibit the two core enzymes that convert tamoxifen to endoxifen, meaning it may reduce the function of tamoxifen. As this research studies human interactions in a very basic form, more clinical research is needed to actually show whether the effect in the body is significant. It may be best to avoid black cohosh if you are taking tamoxifen, and speak to your doctor about alternatives to counteract your hot flashes and other menopausal symptoms. You can also head to our blogs for tips on how to manage hot flashes and tamoxifen side effects.
St. John’s Wort
St. John’s Wort is a herb that is often used to treat depression, anxiety, hot flashes, and sleep issues. It is particularly popular among cancer patients, as it is often seen as a safe natural remedy40,41. However, due to some of its properties, St. John’s Wort may interact with cancer medications and cause them to be less effective. It has been found that St. John’s Wort acts as an inducer of CYP enzymes, particularly the enzyme CYP3A4. In other words, substances within St. John’s Wort can lead to more CYP enzymes and increase their activity42,43,44,45,46.
St. John’s Wort and CYP Enzyme Interactions
As discussed previously, CYP enzymes are key in breaking down tamoxifen into its active form endoxifen. Increasing the number of these enzymes may lead to tamoxifen being cleared out of your body too quickly, before it can have the desired effect on blocking cancer cell growth. Clinical trials have shown that St. John’s Wort interacts with the chemotherapy drugs irinotecan47, imatinib48, and docetaxel49 by reducing the amount of medication circulating in the blood. This was due to the increased CYP3A4 enzymes which cause the chemo-drugs to be removed too quickly50.
It may be best to avoid St. John’s Wort supplements if you are on tamoxifen or discuss this with your doctor. It is notable, for example, that a clinical trial investigating the use of St. John’s Wort to relieve hot flashes in breast cancer patients has been stopped due to “concerns about interaction between St. John’s Wort and tamoxifen.”51.
Ginseng
Ginseng is a supplement which is often taken to alleviate certain cancer treatment side effects, to boost the immune system, or help with anxiety52,53. Ginseng is a strong inhibitor of the enzymes CYP3A4 and CYP2D646,54,55. This means that it stops these enzymes from working, and may affect your body’s ability to process certain drugs which require the use of these enzymes. In one case, the use of ginseng alongside imatinib (a chemotherapy drug) led to liver damage which researchers linked to interactions between ginseng and imatinib56. Due to ginseng’s known inhibition of important CYP enzymes, it is perhaps best to avoid high levels of this supplement whilst taking tamoxifen.
Is there anything that I should eat or drink in moderation while on Tamoxifen?
Some foods are recommended to be taken in moderation while you are on tamoxifen as some studies have shown a link between them and the metabolism of this treatment. However, their effects are small so having these foods once in a while is okay.
Chamomile
Chamomile tea is a soothing drink enjoyed daily by many and has seemingly countless benefits. However, studies have found that in lab experiments, chamomile has the ability to interact and inhibit CYP2D6 and CYP3A4 enzymes 13. This means that theoretically, chamomile could interfere with the conversion of tamoxifen into its active endoxifen form in the body. But there is currently no clinical evidence to show this – there is no doubt that more research on this link needs to be carried out, especially with clinical studies that directly look into whether chamomile could affect the effectiveness of tamoxifen in the body. For now, it is best to consult with your doctor or nutritionist for guidance on whether to start or continue ingesting chamomile whilst taking tamoxifen.
Soy
 Consuming soy remains controversial amongst those diagnosed with breast cancer. The concern often stems from the fact that soy contains what is known as “phytoestrogens” – chemicals which have structures similar to estrogen. Because of the potential for these chemicals to bind to estrogen receptors, it was hypothesised that they could potentially stimulate the growth of ER+ breast cancers 14, 15.
Consuming soy remains controversial amongst those diagnosed with breast cancer. The concern often stems from the fact that soy contains what is known as “phytoestrogens” – chemicals which have structures similar to estrogen. Because of the potential for these chemicals to bind to estrogen receptors, it was hypothesised that they could potentially stimulate the growth of ER+ breast cancers 14, 15.
However, there is actually increasing evidence to suggest that soy consumption may be beneficial in reducing breast cancer risk, mortality and recurrence 14, 16, 17, even whilst on tamoxifen 18. Currently, there are no definitive answers to whether soy should or shouldn’t be included in the diet. With more human clinical studies, our understanding can develop.
There is no clear evidence to suggest that you should stop consuming soy after being diagnosed with breast cancer and whilst taking tamoxifen – it may even be beneficial. So, if you do regularly have soy, or cut it out because you thought it could affect your treatment, feel free to carry on consuming it as part of a well-balanced diet. If you don’t like soy or never included it in your diet before, there’s no need to start now, but if you would like to include it, talk to your doctor or nutritionist who can advise you further.
To learn more about Tamoxifen side effects and how to deal with them, head to our previous blog.
Flaxseed
Like soy, flaxseeds also contain phytoestrogens, which have caused concern for women with breast cancer over the years. However, current evidence seems to suggest that flaxseeds are safe for people who are on tamoxifen. Not only are they safe, but also like soy, flaxseeds may, in fact, be beneficial to those with breast cancer and who are on tamoxifen, with studies suggesting that its consumption may reduce the risk, growth and recurrence of breast cancer 19, 20, 21,22, 23.
Tangerine
Tangerines and other citrus peels contain a chemical called tangeretin, which has been shown to reduce the effectiveness of tamoxifen 24. However, the actual flesh of the fruit contains a lot less tangeretin than the peel, so eating moderate amounts of tangerines and other such citrus fruits should be okay. You may want to be more careful with the amount of marmalade you consume, however, as these tend to include the peel.
Is there anything that I can eat or drink to boost the effectiveness of tamoxifen?
Green tea
 Green tea is a relaxing and comforting drink that pops up time and time again in health forums, with seemingly countless benefits. And it doesn’t stop there – studies have shown that green tea interacts together with tamoxifen, boosting its effectiveness 25,26. The mechanism leading to the beneficial interaction is thought to involve flavonoids (substances with a chemical structure called “phenolic”) such as EGCG (Epigallocatechin gallate) present in green tea.
Green tea is a relaxing and comforting drink that pops up time and time again in health forums, with seemingly countless benefits. And it doesn’t stop there – studies have shown that green tea interacts together with tamoxifen, boosting its effectiveness 25,26. The mechanism leading to the beneficial interaction is thought to involve flavonoids (substances with a chemical structure called “phenolic”) such as EGCG (Epigallocatechin gallate) present in green tea.
Interestingly, these green tea flavonoids have even been reported to reverse the mechanisms by which cancer cells could manage to escape from tamoxifen and become resistant to it 27, 28, 29. Therefore, co-administration of green tea with tamoxifen could also be useful in tamoxifen-resistant breast cancer cases 3.
Which medications should I be aware of on Tamoxifen?
Antidepressants
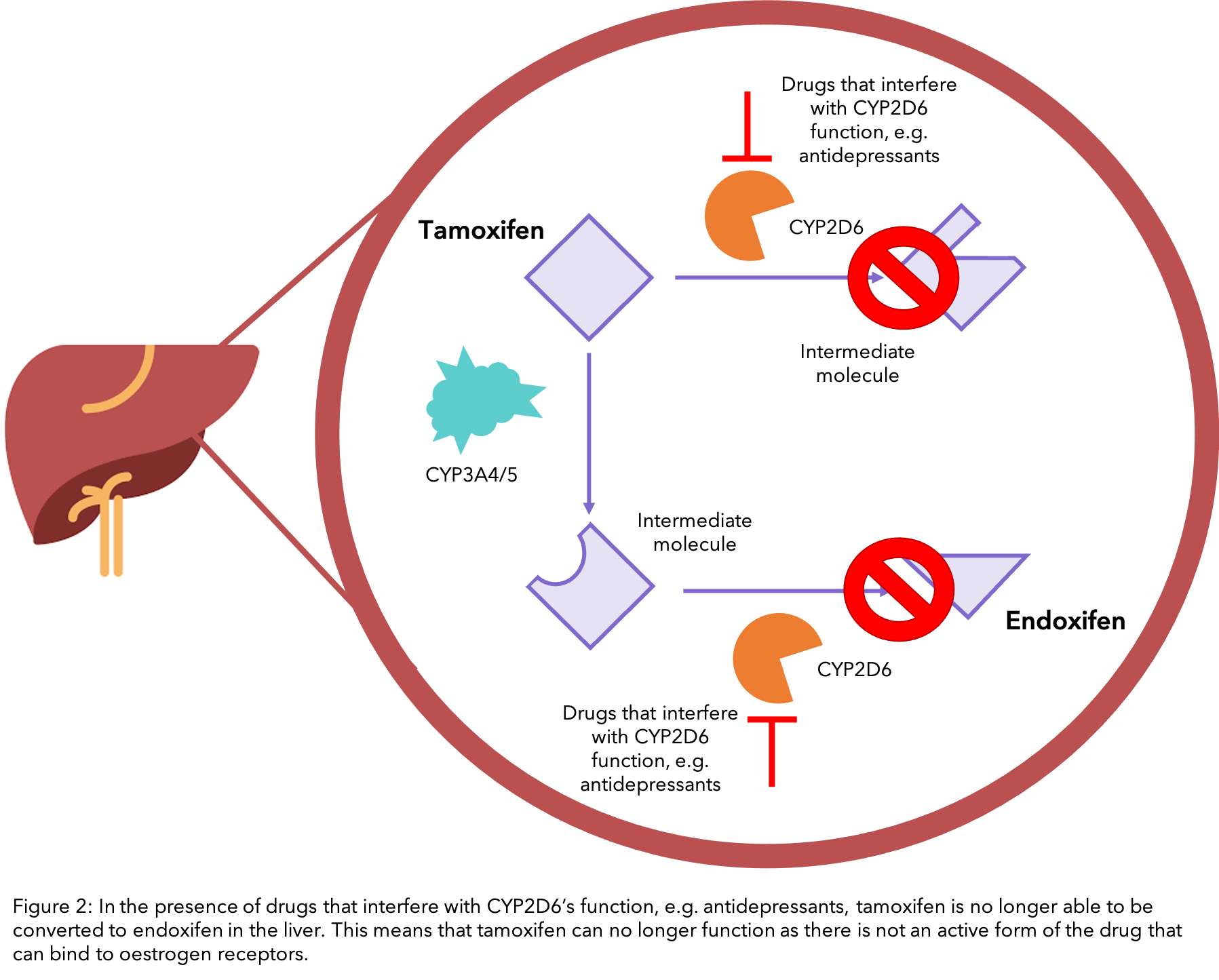
Antidepressive agents are often prescribed to women with breast cancer, not only to counteract depression but also for anxiety and hot flushes 31. However many antidepressants inhibit CYP2D6 to various extents, which can interfere with the conversion of tamoxifen to endoxifen 32, 33,34 (see diagram). In particular, the antidepressants fluoxetine (Prozac® and Sarafem®) and paroxetine have the ability to greatly reduce the effectiveness of tamoxifen as they are strong inhibitors of CYP2D6, therefore they should not be prescribed in conjunction with it.
However, there are safer alternative antidepressants to take whilst on tamoxifen that do not inhibit CYP2D6, such as escitalopram, venlafaxine and mirtazapine 35. Escitalopram (Cipralex® or Lexapro®) is the preferred prescribed antidepressant to breast cancer patients who are on tamoxifen, as it interacts less in the conversion of tamoxifen into endoxifen compared to other antidepressants such as fluoxetine 36. You will likely take on of these three options if you are on tamoxifen and taking prescribed antidepressants. If your antidepressant is not one of the three, it may be worth having a conversation with your oncologist at your next appointment to enquire whether the antidepressant you are on is the best option for you and why.
Antipsychotics
Several antipsychotic medications have also been shown to be inhibitors of CYP2D6 34, 37, 38, 39. In particular of the common antipsychotics, thioridazine, perphenazine and pimozide are the strongest CYP2D6 inhibitors and therefore should not be prescribed alongside tamoxifen. Chlorpromazine, fluphenazine and aloperidol are also CYP2D6 inhibitors, but to a lesser extent. If you have been prescribed any of the antipsychotics mentioned and you are on tamoxifen, you may want to speak to your doctor about whether the antipsychotic you are on is the best option for you – there are several alternatives that are safer to take on tamoxifen that you may want to discuss with them.
Warfarin
The medication warfarin is used as a blood thinner. Warfarin and Tamoxifen are known to interact with each other, leading to an increased risk of bleeding. The exact mechanism of this interaction is unknown, but there have been several cases of bleeding complications in people who are taking both warfarin and Tamoxifen. If these medications are prescribed to the same person, close and constant monitoring is recommended.
The important takeaways
It’s important to note here that most foods and drinks are perfectly fine to have, especially in moderation, and some are even beneficial to ingest whilst on tamoxifen. However, remember that too much of a good thing can sometimes have negative effects so always be careful with taking concentrated supplements and speak to your doctor or cancer nutritionist about your diet before introducing something new or making any drastic changes.
Since most foods are okay in moderation it may be helpful to make notes of what you have eaten on a daily basis – you can do this right in the OWise app! This way, you can keep track of how often you are eating certain foods or drinking certain drinks so that you can plan when to cut back on something you have been having too often, or know when to treat yourself to something you don’t often have.
If you want to learn more about how tamoxifen works, see our previous blog.
References
1. Hansten, P.D., 2018. The underrated risks of tamoxifen drug interactions. European Journal of Drug Metabolism and Pharmacokinetics, 43(5), pp.495-508.
2. Bailey, D.G., Dresser, G. and Arnold, J.M.O., 2013. Grapefruit–medication interactions: Forbidden fruit or avoidable consequences?. Cmaj, 185(4), pp.309-316.
3. Guo, L.Q. and Yamazoe, Y., 2004. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacologica Sinica, 25(2), p.129.
4. Malhotra, S., Bailey, D.G., Paine, M.F. and Watkins, P.B., 2001. Seville orange juice‐felodipine interaction: comparison with dilute grapefruit juice and involvement of furocoumarins. Clinical Pharmacology & Therapeutics, 69(1), pp.14-23.
5. Sharma, R.A., Gescher, A.J. and Steward, W.P., 2005. Curcumin: the story so far. European journal of cancer, 41(13), pp.1955-1968.
6. Song, X., Zhang, M., Dai, E. and Luo, Y., 2019. Molecular targets of curcumin in breast cancer. Molecular medicine reports, 19(1), pp.23-29.
7. Shoba₁, G., Joy₁, D., Joseph₁, T., Rajendran₂, M.M.R. and Srinivas₂, P.S.S.R., 1998. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta medica, 64, pp.353-356.
8. Cho, Y.A., Lee, W. and Choi, J.S., 2012. Effects of curcumin on the pharmacokinetics of tamoxifen and its active metabolite, 4-hydroxytamoxifen, in rats: possible role of CYP3A4 and P-glycoprotein inhibition by curcumin. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 67(2), pp.124-130.
9. Bahramsoltani, R., Rahimi, R. and Farzaei, M.H., 2017. Pharmacokinetic interactions of curcuminoids with conventional drugs: A review. Journal of ethnopharmacology, 209, pp.1-12.
10. Volak, L.P., Ghirmai, S. and Cashman, J.R., 2008. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug metabolism and disposition, 36(8), pp.1594-1605.
11. Hussaarts, K.G., Hurkmans, D.P., Oomen-de Hoop, E., van Harten, L.J., Berghuis, S., van Alphen, R.J., Spierings, L.E., van Rossum-Schornagel, Q.C., Vastbinder, M.B., van Schaik, R.H. and van Gelder, T., 2019. Impact of Curcumin (with or without Piperine) on the Pharmacokinetics of Tamoxifen. Cancers, 11(3), p.403.
12. Fritz, H., Seely, D., McGowan, J., Skidmore, B., Fernandes, R., Kennedy, D.A., Cooley, K., Wong, R., Sagar, S., Balneaves, L.G. and Fergusson, D., 2014. Black cohosh and breast cancer: a systematic review. Integrative Cancer Therapies, 13(1), pp.12-29.
13. Ganzera, M., Schneider, P. and Stuppner, H., 2006. Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life sciences, 78(8), pp.856-861.
14. Nechuta, S.J., Caan, B.J., Chen, W.Y., Lu, W., Chen, Z., Kwan, M.L., Flatt, S.W., Zheng, Y., Zheng, W., Pierce, J.P. and Shu, X.O., 2012. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. The American journal of clinical nutrition, 96(1), pp.123-132.
15. Messina, M.J. and Loprinzi, C.L., 2001. Soy for breast cancer survivors: a critical review of the literature. The Journal of nutrition, 131(11), pp.3095S-3108S.
16. Nachvak, S.M., Moradi, S., Anjom-Shoae, J., Rahmani, J., Nasiri, M., Maleki, V. and Sadeghi, O., 2019. Soy, Soy Isoflavones, and Protein Intake in Relation to Mortality from All Causes, Cancers, and Cardiovascular Diseases: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Journal of the Academy of Nutrition and Dietetics, 119(9), pp.1483-1500.
17. Chi, F., Wu, R., Zeng, Y.C., Xing, R., Liu, Y. and Xu, Z.G., 2013. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev, 14(4), pp.2407-2412.
18. Shu, X.O., Zheng, Y., Cai, H., Gu, K., Chen, Z., Zheng, W. and Lu, W., 2009. Soy food intake and breast cancer survival. Jama, 302(22), pp.2437-2443.
19. Chen, J., Hui, E., Ip, T. and Thompson, L.U., 2004. Dietary flaxseed enhances the inhibitory effect of tamoxifen on the growth of estrogen-dependent human breast cancer (mcf-7) in nude mice. Clinical Cancer Research, 10(22), pp.7703-7711.
20. Flower, G., Fritz, H., Balneaves, L.G., Verma, S., Skidmore, B., Fernandes, R., Kennedy, D., Cooley, K., Wong, R., Sagar, S. and Fergusson, D., 2014. Flax and breast cancer: A systematic review. Integrative cancer therapies, 13(3), pp.181-192.
21. Lowcock, E.C., Cotterchio, M. and Boucher, B.A., 2013. Consumption of flaxseed, a rich source of lignans, is associated with reduced breast cancer risk. Cancer Causes & Control, 24(4), pp.813-816.
22. Chen, J., Power, K.A., Mann, J., Cheng, A. and Thompson, L.U., 2007. Flaxseed alone or in combination with tamoxifen inhibits MCF-7 breast tumor growth in ovariectomized athymic mice with high circulating levels of estrogen. Experimental biology and medicine, 232(8), pp.1071-1080.
23. Saggar, J.K., Chen, J., Corey, P. and Thompson, L.U., 2010. Dietary flaxseed lignan or oil combined with tamoxifen treatment affects MCF‐7 tumor growth through estrogen receptor‐and growth factor‐signaling pathways. Molecular nutrition & food research, 54(3), pp.415-425.
24. Bracke, M.E., Depypere, H.T., Boterberg, T., Van Marck, V.L., Vennekens, K.L.M., Vanluchene, E., Nuytinck, M., Serreyn, R. and Mareel, M.M., 1999. Influence of tangeretin on tamoxifen’s therapeutic benefit in mammary cancer. Journal of the National Cancer Institute, 91(4), pp.354-359.
25. Sartippour, M.R., Pietras, R., Marquez-Garban, D.C., Chen, H.W., Heber, D., Henning, S.M., Sartippour, G., Zhang, L., Lu, M., Weinberg, O. and Rao, J.Y., 2006. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis, 27(12), pp.2424-2433.
26. Shin, S.C. and Choi, J.S., 2009. Effects of epigallocatechin gallate on the oral bioavailability and pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats. Anti-cancer drugs, 20(7), pp.584-588.
27. Farabegoli F, Papi A, Orlandi M, 2011.(-)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep, 31:99-108.
28. Farabegoli F, Papi A, Bartolini G, Ostan R, Orlandi M, 2010.(-)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine;17:356-362.
29. Farabegoli F, Barbi C, Lambertini E, Piva R, 2007. (-)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect Prev, 31:499-540.
30. Yiannakopoulou, E.C., 2014. Interaction of green tea catechins with breast cancer endocrine treatment: a systematic review. Pharmacology, 94(5-6), pp.245-248.
31. Irarrázaval, M.O. and Gaete, L.G., 2016. Antidepressants agents in breast cancer patients using tamoxifen: review of basic and clinical evidence. Revista medica de Chile, 144(10), pp.1326-1335.
32. Jin, Y., Desta, Z., Stearns, V., Ward, B., Ho, H., Lee, K.H., Skaar, T., Storniolo, A.M., Li, L., Araba, A. and Blanchard, R., 2005. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. Journal of the National Cancer Institute, 97(1), pp.30-39.
33. Kelly, C.M., Juurlink, D.N., Gomes, T., Duong-Hua, M., Pritchard, K.I., Austin, P.C. and Paszat, L.F., 2010. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. Bmj, 340, p.c693.
34. Sideras, K., Ingle, J.N., Ames, M.M., Loprinzi, C.L., Mrazek, D.P., Black, J.L., Weinshilboum, R.M., Hawse, J.R., Spelsberg, T.C. and Goetz, M.P., 2010. Coprescription of tamoxifen and medications that inhibit CYP2D6. Journal of Clinical Oncology, 28(16), p.2768.
35. Desmarais, J.E. and Looper, K.J., 2009. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. The Journal of clinical psychiatry, 70(12), pp.1688-1697.
36. Binkhorst L., Bannink, M., de Bruijn, P., Ruit, J., Droogendijk, H., van Alphen, R.J., den Boer, D.T., Ho Lam, M., Jager, A., van Gelder, T. and Mathijssen, R.H.J., 2016. Augmentation of Endoxifen Exposure in Tamoxifen-Treated Women Following SSRI Switch. Clinical Pharmacokinetics, 55(9247), pp.249-255.
37. Otani, K. and Aoshima, T., 2000. Pharmacogenetics of classical and new antipsychotic drugs. Therapeutic drug monitoring, 22(1), pp.118-121.
38. Shin, J.G., Soukhova, N. and Flockhart, D.A., 1999. Effect of antipsychotic drugs on human liver cytochrome P-450 (CYP) isoforms in vitro: preferential inhibition of CYP2D6. Drug metabolism and disposition, 27(9), pp.1078-1084.
39. Desta, Z., Kerbusch, T., Soukhova, N., Richard, E., Ko, J.W. and Flockhart, D.A., 1998. Identification and characterization of human cytochrome P450 isoforms interacting with pimozide. Journal of Pharmacology and Experimental Therapeutics, 285(2), pp.428-437.
40. Hutchinson, E. (2002). Not so innocent. Nature Reviews Cancer, 2(10), pp.720–720. doi:https://doi.org/10.1038/nrc912.
41. Cancer Research UK (n.d.). St John’s wort | Complementary and Alternative therapy | Cancer Research UK. [online] www.cancerresearchuk.org. Available at: https://www.cancerresearchuk.org/about-cancer/treatment/complementary-alternative-therapies/individual-therapies/st-johns-wort.
42. Kober, M., Pohl, K. and Efferth, T. (2008). Molecular Mechanisms Underlying St. Johns Wort Drug Interactions. Current Drug Metabolism, 9(10), pp.1027–1037. doi:https://doi.org/10.2174/138920008786927767.
43. Caraci, F., Crupi, R., Drago, F. and Spina, E. (2011). Metabolic Drug Interactions Between Antidepressants and Anticancer Drugs: Focus on Selective Serotonin Reuptake Inhibitors and Hypericum Extract. Current Drug Metabolism, 12(6), pp.570–577. doi:https://doi.org/10.2174/138920011795713706.
44. Whitten, D.L., Myers, S.P., Hawrelak, J.A. and Wohlmuth, H. (2006). The effect of St John’s wort extracts on CYP3A: a systematic review of prospective clinical trials. British Journal of Clinical Pharmacology, 62(5), pp.512–526. doi:https://doi.org/10.1111/j.1365-2125.2006.02755.x.
45. Moore, L.B., Goodwin, B., Jones, S.A., Wisely, G.B., Serabjit-Singh, C.J., Willson, T.M., Collins, J.L. and Kliewer, S.A. (2000). St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proceedings of the National Academy of Sciences, 97(13), pp.7500–7502. doi:https://doi.org/10.1073/pnas.130155097.
46. Graça, C., Cupido, M., Tavares, R. and Consul, R. (2018). Clinical Outcomes from Tamoxifen Drug-herb Interactions. International Journal of Clinical Pharmacology & Pharmacotherapy, 3(2). doi:https://doi.org/10.15344/2456-3501/2018/140.
47. Mathijssen, R.H.J. (2002). Effects of St. John’s Wort on Irinotecan Metabolism. CancerSpectrum Knowledge Environment, 94(16), pp.1247–1249. doi:https://doi.org/10.1093/jnci/94.16.1247.
48. FRYE, R., FITZGERALD, S., LAGATTUTA, T., HRUSKA, M. and EGORIN, M. (2004). Effect of St John’s wort on imatinib mesylate pharmacokinetics. Clinical Pharmacology & Therapeutics, 76(4), pp.323–329. doi:https://doi.org/10.1016/j.clpt.2004.06.007.
49. Goey, A.K.L., Meijerman, I., Rosing, H., Marchetti, S., Mergui-Roelvink, M., Keessen, M., Burgers, J.A., Beijnen, J.H. and Schellens, J.H.M. (2013). The Effect of St John’s Wort on the Pharmacokinetics of Docetaxel. Clinical Pharmacokinetics, 53(1), pp.103–110. doi:https://doi.org/10.1007/s40262-013-0102-5.
50. de Jong, F.A., Sparreboom, A., Verweij, J. and Mathijssen, R.H.J. (2008). Lifestyle habits as a contributor to anti-cancer treatment failure. European Journal of Cancer, 44(3), pp.374–382. doi:https://doi.org/10.1016/j.ejca.2007.12.012.
51. Clinical Trials (n.d.). ClinicalTrials.gov. [online] clinicaltrials.gov. Available at: https://clinicaltrials.gov/study/NCT00110136 [Accessed 18 Jul. 2024].
52. Chen, S., Wang, Z., Huang, Y., O’Barr, S.A., Wong, R.A., Yeung, S. and Chow, M.S.S. (2014). Ginseng and Anticancer Drug Combination to Improve Cancer Chemotherapy: A Critical Review. Evidence-Based Complementary and Alternative Medicine, [online] 2014, pp.1–13. doi:https://doi.org/10.1155/2014/168940.
53. PDQ Integrative, Alternative, and Complementary Therapies Editorial Board (2002). Cancer Therapy Interactions With Foods and Dietary Supplements (PDQ®): Health Professional Version. [online] PubMed. Available at: https://www.ncbi.nlm.nih.gov/books/NBK563071/.
54. Wanwimolruk, S., Phopin, K. and Prachayasittikul, V., 2014. Cytochrome P450 enzyme mediated herbal drug interactions (Part 2). EXCLI Journal, 13, pp.869-896
55. Yang, L., Wang, Y., Xu, H., Huang, G., Zhang, Z., Ma, Z. and Gao, Y. (2019). Panax ginseng Inhibits Metabolism of Diester Alkaloids by Downregulating CYP3A4 Enzyme Activity via the Pregnane X Receptor. Evidence-Based Complementary and Alternative Medicine, [online] 2019, p.e3508658. doi:https://doi.org/10.1155/2019/3508658.
56. Bilgi, N., Bell, K., Ananthakrishnan, A.N. and Atallah, E. (2010). Imatinib and Panax ginseng: a potential interaction resulting in liver toxicity. The Annals of Pharmacotherapy, [online] 44(5), pp.926–928. doi:https://doi.org/10.1345/aph.1M715.
