
“I want to be defined by what I am, not by what I am not” – Dr Steven Isakoff (Breast Oncologist) on Triple Negative Breast Cancer
It’s time to focus on Triple Negative Breast Cancer (TNBC). As summed up by Dr Isakoff, TNBC has been defined by not what it is, but what it isn’t. So what is it not? TNBC means the growth of the cancer does not involve estrogen, progesterone (hormones often associated with breast cancer) receptors and HER2 receptors. Most recent treatments have been developed for breast cancer with these receptors. This has meant TNBC has had limited treatment options, namely surgery and chemotherapy. However, recent developments in research have allowed for a better understanding of TNBC and opened doors for new treatment options.
“From my perspective, this means there is a huge amount of hope. We are clearly improving the treatments in early TNBC. And it clearly seems to me we are improving outcomes and cure rates.” – Dr Peter Schmid, Chair in cancer medicine at Barts Cancer Institute, Queen Mary University of London.
Here we are going to explain the characteristics of triple negative disease, the treatments that are commonly used, as well as newly approved treatments and potential therapies currently undergoing clinical trials.
How common is TNBC?
TNBC represents approximately 10-15% of all diagnosed breast cancers1,2, with over 30,000 cases diagnosed every year in the US3. This type of breast cancer seems to be more common in African4,5 and Latina women6, as well as in women under 407,8. The incidence of TNBC differs per state economic area (SEA). The adjusted TNBC incidence rate for the overall population of the US between 2011 and 2013 was 13.7 cases per 100,000 women per year. The map below shows the incidence rate per SEA35.
For more information about familiar risk and breast cancer genetic mutations, head to our previous blog on BRCA.
What can your pathology report tell you about TNBC?
The analysis of your tissue sample from a biopsy or surgery will indicate that your tumor does not express estrogen receptor (ER), progesterone receptor (PR) or human epidermal growth factor 2 (HER2). In your pathology results, you may read about the percentage of cells being positive for estrogen and progesterone. If this percentage is less than 1%, the cancer is considered to be hormone receptor-negative. For the HER2 protein, the result would be rated as 0, 1+, 2+ to be considered HER2-negative12.
Triple negative cancer cells tend to look very different from normal breast cancer cells, which means that the cancer is classified as high grade (e.g. grade 3). Cells that behave in this way tend to have high levels of markers that indicate cell proliferation such as Ki67, p53+ and p63+. This means that the cells grow and divide quickly13.
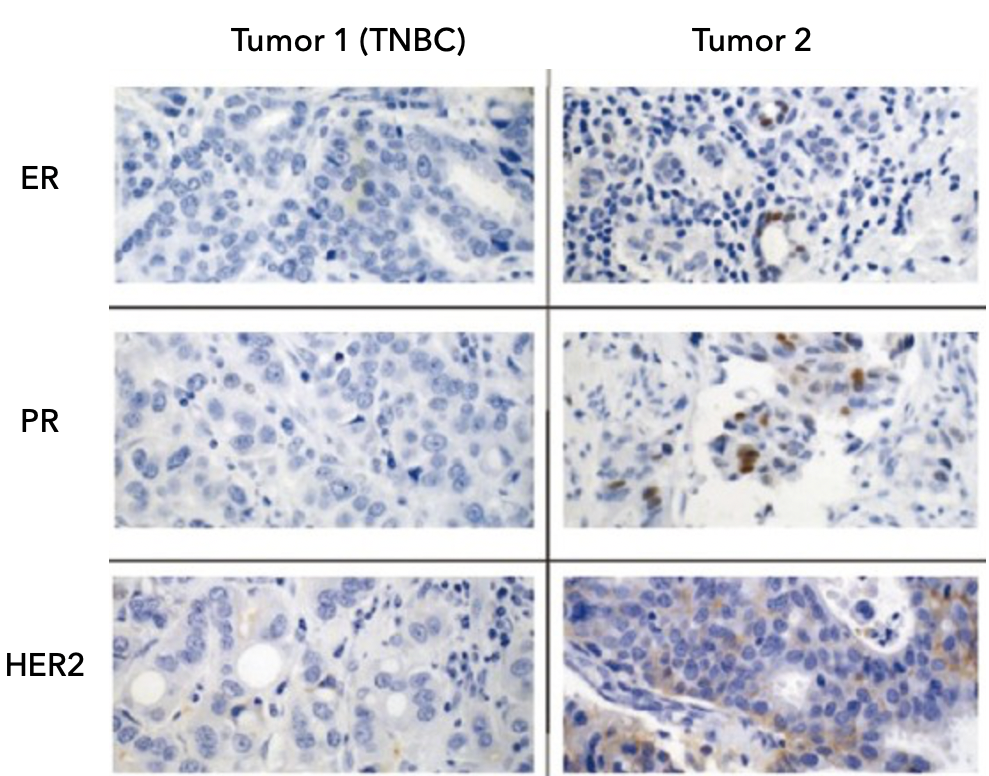
Figure 1. This is an example of stained breast tissue. The brown color indicated the presence of estrogen (ER), progesterone (PR), and HER2 receptors. Tumor 1 is TNBC and tumor 2 is positive for ER, PR, and HER2. Image modified from Adisa et al, 201234
Testing of the protein PD-L1 (anti-programmed cell death-ligand 1) is approved for TNBC patients. This protein is located on the surface of some cancer cells. PD-L1 protein is recognized by a receptor (PD-1) on the surface of a type of immune cell (T-cell). The immune system is the defense system of the body that protects us against pathogens and disease. Recent research has shown that if PD-L1 is blocked, these immune cells will recognize cancer cells as foreign and would attack them15. Triple negative patients that are PD-L1 positive may benefit from newly developed therapies (see section below).
To better understand your pathology results, head to our previous pathology report blog.
Treatment plan
We have pulled together all the different treatment options for triple negative breast cancer that are available in the United States. We have classified them into three types: chemotherapy, PARP inhibitors and immunotherapy. In this blog, we are going to identify them all, explain how each work and whether or not they have been approved by the US Food and Drug Administration
The Food and Drug Administration (FDA) is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of our nation’s food supply, cosmetics, and products that emit radiation.16
Chemotherapy
Chemotherapy is a treatment that uses anti-cancer drugs to target and kills fast-dividing cells in the body. It works by interfering with the cancer cells’ ability to divide and grow. It can be provided before surgery (neoadjuvant) or after surgery (adjuvant).
Chemotherapy before surgery
Neoadjuvant chemotherapy can either be anthracycline- or taxane-based chemotherapy. In addition to either of these, platinum chemotherapy is usually added to the regimen36.
- Platinum chemotherapy drugs include cisplatin, carboplatin and oxaliplatin.
- Taxane chemotherapy drugs include paclitaxel (Taxol®) and docetaxel (Taxotere®).
- Anthracyclines chemotherapy drugs include doxorubicin (Adriamycin®), epirubicin (Ellence®), doxorubicin (Doxil®), daunorubicin (Cerubidine®), mitoxantrone (Novantrone®).
This therapy can reduce the size of cancer and improve the chances of cancer disappearing in both the breast and lymph nodes in the armpit. However, platinum-based chemotherapy can produce severe side effects. This includes anemia which means you have a low number of red blood cells and neutropenia which indicates that you have a low number of neutrophils (immune cells that defend you from viruses and bacteria). It is possible that patients with additional medical conditions receive adjuvant chemotherapy (after surgery) as there is a lower risk of developing these side effects since other chemotherapy drugs are used11.
Chemotherapy after surgery
The adjuvant chemotherapy regimen recommended by the NHS includes both a taxane and an anthracycline drug11. Taxane chemotherapy drugs include paclitaxel (Taxol®), nab-paclitaxel (Abraxane®) and docetaxel (Taxotere®).
PARP inhibitors
PARP (poly (ADP-ribose) polymerase) is a protein that helps both healthy and cancer cells repair DNA damage, allowing cells to survive and continue to function. PARP inhibitors work by blocking PARP proteins in cancer cells. This prevents them from repairing DNA damage, and often leads to cell death18.
PARP inhibitors are a therapy for those with BRCA genetic mutations, which are common among TNBC patients. This is because PARP inhibitors are able to cause the death of cells carrying these genetic mutations. Two of the main PARP inhibitors are Olaparib (Lynparza®) and Talazoparib (Talzenna®). In the US both have been licensed for both early-stage and metastatic BRCA-positive disease20.
Immunotherapy
Immunotherapy uses the immune system to fight cancer. Therefore, it works by helping the immune system recognize and attack cancer cells. Some types of immunotherapy are also called targeted treatments or biological therapies.
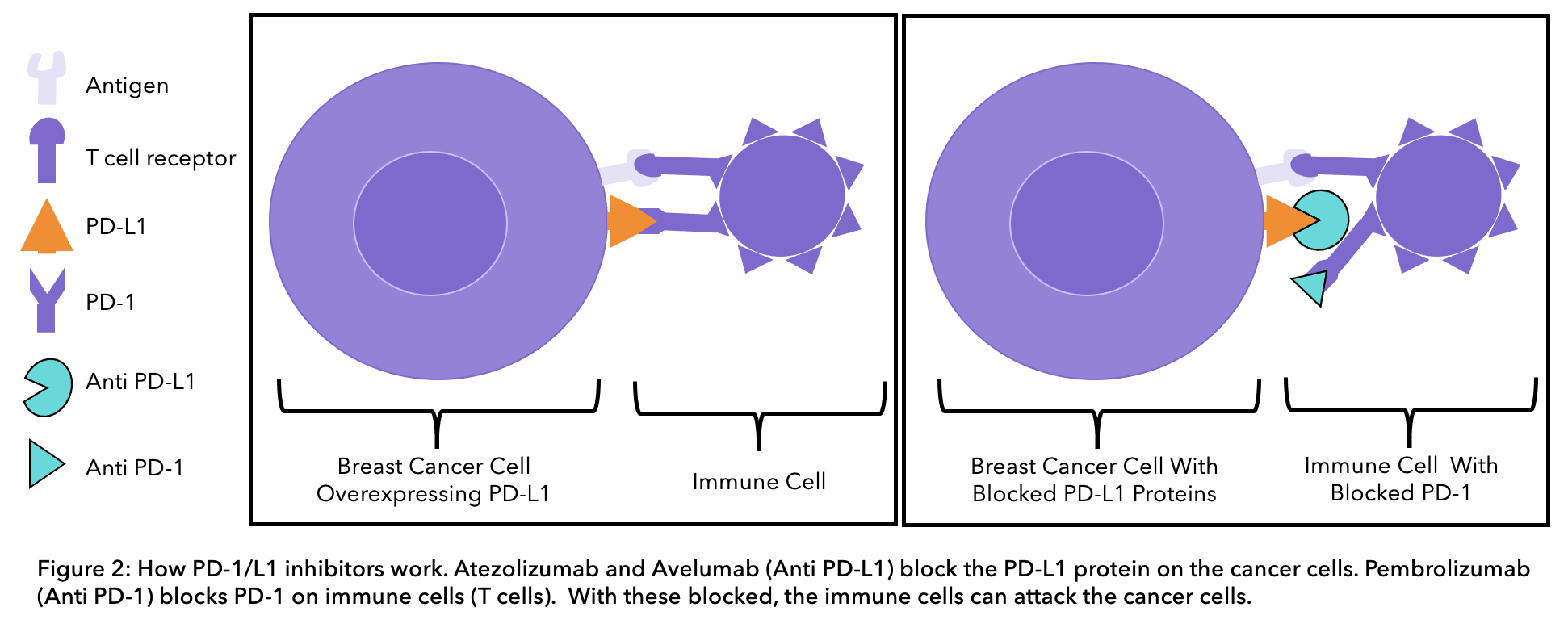
The PD-1 protein is a receptor on the surface of a type of immune cell, called a T-cell (see figure 2 below). Cells in the body have the PD-L1 protein on their surface, this binds to the PD-1 receptor on immune cells and prevents immune cells from attacking human cells. In some cases, cancer cells can have the PD-L1 protein on their surface. The PD-L1 protein on cancer cells can bind PD-1 receptors on immune cells, enabling them to trick the body into thinking that they are normal cells and preventing the immune system from attacking them. Current immunotherapy treatments for TNBC can either block the PD-L1 protein on cancer cells or the PD-1 receptor on immune cells to stop the two from binding.
Pembrolizumab (Keytruda®)
Pembrolizumab blocks the action of the PD-1 protein on immune cells, enabling the immune system to recognize and kill cancer cells. Pembrolizumab is approved to treat early TNBC patients at a high risk of recurrence. High risk means either the tumor size is 1-2cm in diameter with positive lymph nodes or the tumor size is over 2cm in diameter with or without positive lymph nodes. The KEYNOTE-522 study compared treatment before and after surgery with pembrolizumab and chemotherapy or chemotherapy alone. Results showed that the cancer did to come back or spread in 84.5% of patients on pembrolizumab and chemotherapy, compared to 76.8% on chemotherapy alone26.
To be eligible for treatment the tumor needs to have enough of the PD-L1 protein on its surface. In this case, the tumor must have a combined positive score > 10. Pembrolizumab can be given as neoadjuvant treatment (before surgery) with chemotherapy and then as adjuvant treatment (after surgery) alone27.
Atezolizumab (Tecentriq®) with nab-paclitaxel (Abraxane®)
Atezolizumab blocks the PD-L1 protein on cancer cells, preventing it from binding to the PD-1 receptor on immune cells. This enables the immune system to recognize and kill cancer cells. It is approved in combination with the chemotherapy drug nab-paclitaxel (Abraxane®) to treat TNBC that is locally advanced (stage 3), cannot be operated on or is metastatic. This therapy can increase overall survival by around 9.5 months of metastatic or locally inoperable TNBC patients that are PD-L1-positive15. Immune cell staining must be done to determine if the tumor has enough of the PD-L1 protein on its surface. To be eligible for treatment immune cell staining needs to be > 1%.
Antibody-drug conjugate (ADC)
Sacituzumab govitecan (Trodelvy®) is a promising type of drug called an antibody-drug conjugate (ADC), which means it is made up of a monoclonal antibody linked to a chemotherapy drug. In this case, it is made up of the sacituzumab monoclonal antibody, linked to the chemotherapy drug SN-38. Sacituzumab targets the TROP-2 protein, which is a new protein found in abundance in this type of cancer29. When sacituzumab targets and binds to the cell the chemotherapeutic becomes active, killing the cell. This therapy has now been approved for use as a third line treatment option for TNBC that is locally advanced (stage 3), cannot be operated on, or is metastatic.
Research and new treatments
Research has shown that TNBC is not a single subtype. Recent studies are showing that triple-negative breast cancer can be divided into different groups depending on the molecules/proteins cells express. In fact, triple negative breast cancers are very heterogeneous and have diverse characteristics. For example, using gene analyses, some research groups have identified six different TNBC subtypes: two basal-like (BL1 and BL2), an immunomodulatory (IM), a mesenchymal (M), a mesenchymal stem–like (MSL), and a luminal androgen receptor (LAR) subtype 28 . These types of classifications are key for finding new therapeutic routes.
Durvalumab (Imfinzi®) is currently approved to treat non-small cell lung cancer and is being trailed for use in breast cancer. This drug is a type of immunotherapy that blocks the PD-L1 protein on cancer cells. The GeparNuevo clinical trial studied this through treating early TNBC patients with durvalumab combined with chemotherapy or chemotherapy alone. Results showed that after 3 years, 92.5% of patients on durvalumab and chemotherapy were alive, compared to 83.5% on chemotherapy alone 35. This highlights a potential treatment option for early TNBC patients that may become available in the future.
Common side effects
Chemotherapy targets all fast-dividing cells in your body. As cancer cells are fast dividing, it is particularly these cells that are targeted and destroyed. But other fast-dividing cells are also affected, including hair follicles, the lining of the mouth, skin, nails and intestines. That’s why hair loss, brittle nails and diarrhea are very common side-effects. Other very common side effects are sickness and vomiting.
Platinum-based chemotherapy may have severe side effects such as the risk of infection due to a drop in white blood cells (neutropenia), breathlessness and looking pale due to a drop in red blood cells (anemia) and bruising (due to a drop of platelets in your blood). Other platinum side effects include fatigue, feeling sick, loss of appetite, diarrhea, kidney damage, changes to your hearing and to the levels of minerals in your blood 30.
Anthracyclines’ side effects include vomiting, nausea and alopecia. However, it can also cause heart function problems. This is why you might have heart tests to check the health of your heart before and during chemotherapy31.
Some common side effects of Atezolizumab (Tecentriq®) are loss of appetite, difficulty breathing, fatigue, diarrhea, feeling sick, skin changes, joint or back pain and urinary tract infections32. Lastly, the main side effects of PARP inhibitors are increased risk of infection, diarrhea, feeling sick, indigestion, headaches and changes to how your kidney and/or liver works33.
Do you know that you can track your side effects with OWise? With over 30 side effects and symptoms to choose from, you can track any changes and share these with your care team and loved ones. Better communication with your care team can make sure you receive the best care possible.
And that’s all of the TNBC treatments summed up
We hope that you now better understand TNBC, the treatment options and that you can feel confident in discussions with your care team. Our aim is to keep you informed with personalized information. You can access this by following our Instagram, Facebook, or downloading OWise.
References
- Lund, M., Trivers, K., Porter, P., Coates, R., Leyland-Jones, B., Brawley, O., Flagg, E., O’Regan, R., Gabram, S. and Eley, J., 2008. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Research and Treatment, 113(2), pp.357-370.
- Plasilova, M., Hayse, B., Killelea, B., Horowitz, N., Chagpar, A. and Lannin, D., 2016. Features of triple-negative breast cancer:: Analysis of 38,813 cases from the national cancer database. Medicine, 95(35), p.e4614.
- American Cancer Society. www.cancer.gov, 2022. Triple Negative Breast Cancer [online] Available at: <https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html> [Accessed 10 October 2022].
- Brewster, A., Chavez-MacGregor, M. and Brown, P., 2014. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. The Lancet Oncology, 15(13), pp.e625-e634.
- Dindyal, S., Ramdass, M. and Naraynsingh, V., 2008. Early onset breast cancer in black British women: a letter to the editor of British Journal of Cancer regarding early onset of breast cancer in a group of British black women. British Journal of Cancer, 98(8), pp.1482-1482.
- Rey-Vargas, L., Sanabria-Salas, M., Fejerman, L. and Serrano-Gómez, S., 2019. Risk Factors for Triple-Negative Breast Cancer among Latina Women. Cancer Epidemiology Biomarkers & Prevention, 28(11), pp.1771-1783.
- Keegan, T., DeRouen, M., Press, D., Kurian, A. and Clarke, C., 2012. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Research, 14(2).[8] Siddharth, S. and Sharma, D., 2018. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers, 10(12), p.514.
- Siddharth, S. and Sharma, D., 2018. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers, 10(12), p.514.
- Pogoda, K., Niwińska, A., Sarnowska, E., Nowakowska, D., Jagiełło-Gruszfeld, A., Siedlecki, J. and Nowecki, Z., 2020. Effects of BRCA Germline Mutations on Triple-Negative Breast Cancer Prognosis. Journal of Oncology, 2020, pp.1-10.
- Ellsworth, D., Turner, C. and Ellsworth, R., 2019. A Review of the Hereditary Component of Triple Negative Breast Cancer: High- and Moderate-Penetrance Breast Cancer Genes, Low-Penetrance Loci, and the Role of Nontraditional Genetic Elements. Journal of Oncology, 2019, pp.1-10.
- Nice.org.uk. 2020. Early And Locally Advanced Breast Cancer: Diagnosis And Management. [online] Available at: <https://www.nice.org.uk/guidance/ng101/resources/early-and-locally-advanced-breast-cancer-diagnosis-and-management-pdf-66141532913605> [Accessed 9 October 2020].
- Cancer.org. 2020. Breast Cancer HER2 Status | HER2-positive Breast Cancer. [online] Available at:https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-her2-status.html [Accessed 7 October 2020].
- Badve, S., Dabbs, D., Schnitt, S., Baehner, F., Decker, T., Eusebi, V., Fox, S., Ichihara, S., Jacquemier, J., Lakhani, S., Palacios, J., Rakha, E., Richardson, A., Schmitt, F., Tan, P., Tse, G., Weigelt, B., Ellis, I. and Reis-Filho, J., 2010. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Modern Pathology, 24(2), pp.157-167.
- Nice.org.uk. 2020. Atezolizumab With Nab-Paclitaxel For Treating PD-L1-Positive, Triple-Negative, Advanced Breast Cancer. [online] Available at: <https://www.nice.org.uk/guidance/ta639/documents/final-appraisal-determination-document> [Accessed 9 October 2020].
- Schmid, P., Rugo, H., Adams, S., Schneeweiss, A., Barrios, C., Iwata, H., Diéras, V., Henschel, V., Molinero, L., Chui, S., Maiya, V., Husain, A., Winer, E., Loi, S. and Emens, L., 2020. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology, 21(1), pp.44-59.
- US Food & Drug Administration, 2022, FDA [online] Available at: https://www.fda.gov/about-fda/what-we-do [Accessed 10 October 2022].
- Nice.org.uk. 2020. NHS England interim treatment options during theCOVID-19 pandemic [online]. Available at: https://www.nice.org.uk/guidance/ng161/resources/interim-treatment-change-options-during-the-covid19-pandemic-endorsed-by-nhs-england-pdf-8715724381
- Cancerresearchuk.org. 2020. Olaparib (Lynparza) | Cancer Information | Cancer Research UK. [online] Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/olaparib-lynparza [Accessed 12 October 2020].
- Nice.org.uk. 2020. Project Information | Olaparib For Treating BRCA 1 Or 2 Mutated Metastatic Breast Cancer After Prior Chemotherapy [ID1382] | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta10342 [Accessed 12 October 2020].
- Nice.org.uk. 2020. Project Information | Talazoparib For Treating BRCA 1 Or 2 Mutated Advanced Breast Cancer After Prior Chemotherapy [ID1432] | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta10366 [Accessed 12 October 2020].
- breastcancernow.org. 2020. The new Cancer Drug Fund. [online]. Available at https://breastcancernow.org/about-us/news-personal-stories/new-cancer-drugs-fund [Accessed 12 October 2020].
- Nice.org.uk. 2020. Improved deal means new treatment for a type of advanced breast cancer can be recommended by NICE. [online] Available at:https://www.nice.org.uk/news/article/improved-deal-means-new-treatment-for-a-type-of-advanced-breast-cancer-can-be-recommended-by-nice [Accessed 12 October 2020].
- Nice.org.uk. 2020. Atezolizuman with nab-paclitaxel for untreated PD-L1-positive, locally advanced or metastatic, triple negative breast cancer. [online] Available at:https://www.nice.org.uk/guidance/TA639 [Accessed 12 October 2020].
- Adams S, Schmid P, Rugo HS, Winer, EP, Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, Hui R, Curigliano G, O’Shaughnessey JO, Loi S, Paluch-Shimon, S, Tan AR, Card D, Zhao J, Karantza V, Cortes J. 2019. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort a of the phase 2 KEYNOTE-086 study. Annals of Oncology. 30(3):397–404.
- Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Hendrik-Tobias A, Forero-Torres A, Boccia R, Lippman M, Somer R, Smakal M, Emens L, Hrinczencki B, Edenfield W, Gurtler J, Von Heydebreck A, Grote HJ, Chin K, Hamilton E. 2018. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 167:671–86.
- Schmid P, Cortes K, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Hee Park Y, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J. 2020. Pembrolizumab for Early Triple-Negative Breast Cancer. The New England Journal of Medicine. 382:810-821.
- Nice.org.uk. 2020. Health Technology appraisal. Pembrolizumab in combination with chemotherapy for neoadjuvant treatment of triple negative breast cancer [online]. Available at: https://www.nice.org.uk/guidance/gid-ta10399/documents/draft-scope-post-referral[Accessed 12 October 2020].
- Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. 2014. New strategies for triple negative breast cancer- deciphering the heterogeneity. Clinical Cancer Research. 20(4):782-790.
- pharmaphorum.com. 2020. Immunomedics wows ESMO with breast cancer data reveal [online]. Available at: https://pharmaphorum.com/news/immunomedics-wows-esmo-with-breast-cancer-data-reveal/ [Accessed 12 October 2020].
- Cancerresearchuk.org. 2020. Cisplatin [online]. Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/cisplatin [Accessed 12 October 2020].
- Cancerresearchuk.org. 2020. Doxorubicin [online]. Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/doxorubicin [Accessed 12 October 2020].
- Cancerresearchuk.org. 2020. Atezolizumab (Tecentriq). [online]. Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/atezolizumab [Accessed 12 October 2020].
- Cancerresearchuk.org. 2020. PARP inhibitors [online]. Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/targeted-cancer-drugs/types/PARP-inhibitors [Accessed 12 October 2020].
- Adisa CA, Eleweke N, Alfred AA, Campbell MJ, Sharma R, Nseyo O, Tandon V, Mukhtar R, Greningen A, Di Risi J, Esserman LJ. 2012. Biology of breast cancer in Nigerian women: a pilot study. Annals of African Medicine. 11(3)):169-175.
- Han, Hyo S, et al. “Early-Stage Triple-Negative Breast Cancer Journey: Beginning, End, and Everything in Between.” American Society of Clinical Oncology Educational Book, no. 43, 1 June 2023, https://doi.org/10.1200/edbk_390464.
- Breast Cancer Invasive 2020 Available Online at NCCN.org/Patients NCCN GUIDELINES for PATIENTS. Apr.
